


ReFoam™ is an innovative foam scaffold for treating periodontal disease and filling bone defects
ReFoam™ is a biocompatible and biodegradable scaffold designed for filling and reconstructing bone defects in the maxillofacial complex.
It features a three-dimensional foam scaffold that supports tissue repair and recovery.
With an eye to the dental clinician’s needs, we are developing a versatile product line to support the various clinical indications.
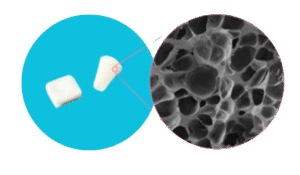
CellFoam™.Inside.
CellFoam™ technology is a protein-based foam scaffold designed to support the body’s natural tissue healing process. It helps the body do what it does best, to heal. When applied into damaged tissue, CellFoam™ promotes natural tissue repair and effectively helps repair anatomical defects. The scaffold naturally degrades in about 3-5 months, while a new healthy tissue takes place.
It's easy to use ReGum™Vet: only 4 simple steps

Step 1
Soak ReGum™ Vet
to reconstitute elasticity

Step 2
Trim ReGum™ Vet
to fit surgical site

Step 3
Place ReGum™ Vet
in the surgical site

Step 2
Close the surgical site
Nothing motivates us more than seeing real results
Don't take our word for it, see what clinicians have to say



ReFoam™ Benefits
ReFoam™ provides a comprehensive solution for the treatment of periodontitis, a condition that affects 40%-70% of adults. This advanced foam scaffold supports tissue growth, including bone and soft tissue, through a single procedure. Unlike existing market options, it effectively addresses all layers impacted by the disease.
Key advantages
- Enables significant vertical and horizontal bone recovery
- Stimulates recovery of both bone and soft tissue synchronously
- Eliminates the need for additional GTR membranes
- Reduces the need for repeated future procedures
- Biodegradable & biocompatible.
- Safe
- Easy to use
FAQ
What is ReFoam™?
ReFoam™ is an innovative foam-based scaffold designed for the repair of bony defects and repair of damaged dental tissues in humans. It is based on BioChange’s proprietary CellFoam™ technology.
What are the uses of ReFoam™?
ReFoam™ is being developed to treat periodontal disease, a widespread condition affecting 40–70% of adults over 30. It aims to restore bone and soft tissues, including functional and structural, offering a comprehensive tissue repair solution.
Is ReFoam™ already approved for clinical use?
Not yet. ReFoam™ is currently undergoing regulatory review, the clearance for human dental use is expected by mid-2026.
Has ReFoam™ been tested or used before?
A veterinary version of the product has already been successfully launched, demonstrating strong clinical and commercial results with over 40k unit deployed (as of mis 2025). This serves as a robust proof of safety and efficacy and supports the ongoing development of the human version.
In what sizes will ReFoam™ be available?
ReFoam™ will be launched in multiple sizes to accommodate various clinical needs. Specific configurations will be announced closer to the official launch.
When will ReFoam™ be available for purchase?
The expected launch date is mid-2026, pending regulatory clearance.
Where can I get more information or updates on ReFoam™?
Visit www.biochange.life for the latest updates, or contact our team directly at info@biochange.life
Are you open for business collaboration or a global distribution partnership?
We are always looking for new business opportunities, for more info, please contact :Hilit Hochman, VP Sales& Marketing at BioChange: hilit@BioChange.life
Grow younger with us
Join our community and stay up to date with the latest advancements. We are committed to providing you with valuable information about our innovations.
By clicking Subscribe, you agree to our Terms and Conditions.
